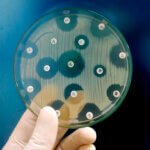
EN 1040 is an internationally known test standard, officially recognized as BS EN 1040:2005. It is intended to evaluate the basic bactericidal activity of chemical disinfectants by using a quantitative suspension test. It is a critical test for manufacturers to verify whether their products can effectively reduce bacteria presence before reaching the market, thus meeting safety and efficacy standards. It also helps manufacturers understand regulatory requirements and ensures that their products are safe and efficacious for use within various sectors such as hospitals, schools, and homes.
EN 1040 test conditions and requirements
Test organisms
- Staphylococcus aureus (ATCC 6538P) and Pseudomonas aeruginosa (ATCC 15442) are the mandatory test organisms.
Test temperature
- A temperature of 20°C should be maintained to resemble room conditions.
Contact time
- A contact time of 5 minutes is specified by the standard to evaluate the efficacy of the disinfectant over different periods. Additional contact times may be chosen from 1 min, 15 min, 30 min or 60 min as per the manufacturer’s request.
Passing criteria
- A disinfectant must demonstrate a minimum of a 5-log reduction in bacterial count under controlled laboratory conditions to be considered effective as per EN 1040 standard.
Test procedure for EN 1040 test
EN 1040 describes a test method developed for the assessment of the bactericidal activity of chemical disinfectants and antiseptics. This method involves a number of stages starting from the preparation of the bacterial strains to the final analysis of the results obtained in the test. Understanding each component is necessary to verify that the products meet the respective bactericidal efficacy standards.
Preparing test solutions
- The chemical disinfectant or antiseptic under test is prepared at various concentrations, generally not exceeding 80% of the original concentration due to the dilution effect when adding the bacterial suspension and water. It’s essential that these preparations are homogeneous and stable under test conditions.
Inoculation and exposure
- The test involves challenging the known volume of bacterial suspension with disinfectant. The exact volume and concentration ratios are important and must adhere to the specifications mentioned in the standard. This mixture is then incubated at a controlled temperature for a defined contact time.
Neutralization and recovery
- Immediately after the exposure period, the reaction is halted by the addition of a neutralizing agent. This step is very important as it stops the action of the disinfectant, allowing for an accurate assessment of the efficacy of disinfectants. The choice of neutralizer depends on the disinfectant’s chemical nature and must be validated to ensure that neutralization happens effectively and quickly neutralizes the antimicrobial activity without harming the bacteria.
Enumerating viable organisms
- After neutralization, the number of viable bacteria is quantified using a plate count method. This involves plating the neutralized mixture on a growth medium and incubating it under suitable conditions to allow any surviving bacteria to form colonies. The colonies are counted to determine the number of viable bacterial units.
Calculating bactericidal activity
- The effectiveness of the disinfectant is calculated in terms of log reduction in the number of viable bacteria. A minimum of a 5-log reduction (99.999% kill) is required for a product to pass the test.
It is important that manufacturers and laboratories be aware of the EN 1040 standard for the development and validation of effective products with antimicrobial properties. Being in compliance with these standards contributes to public health safety and compliance with regulatory authorities.
The role of antimicrobial testing labs
Provides Expertise in Microbial Testing
- Testing labs have the expertise and infrastructure required to perform accurate tests. Thus they play an important role in conducting EN 1040 tests.
Contributes to Product Development
- These labs can help manufacturers during product development stages by conducting initial tests of the product formulations. The results from such initial tests can be used by manufacturers to refine their products to meet the required claims and quality.
Assurance of Public Safety
- Testing laboratories help ensure that only effective and reliable disinfectants reach the market by testing the products thoroughly to meet the EN 1040 standard, thus protecting public health.
At MIS, we conduct the EN 1040 test for chemical disinfectants and antiseptic formulations used in various sectors. Our microbiology experts utilize both standardized and customized methods to meet the specific testing needs of our clients’ product samples.
Contact us today to receive a quote for EN 1040 or to learn more about our microbiology testing services. Our team of microbiology experts is ready to provide you with detailed consultation and guidance tailored to your testing requirements.