- Swiss testing laboratory
BS EN 14348 : 2005
Quantitative suspension test for the evaluation of mycobactericidal activity of chemical disinfectants in the medical area including instrument disinfectants (phase 2, step 1)

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Related tests for you
Quick understanding of the test
BS EN 14348 : 2005 - Quantitative suspension test for the evaluation of mycobactericidal activity of chemical disinfectants in the medical area including instrument disinfectants (phase 2, step 1)
- Mycobacterium avium
- Mycobacterium terrae
- The test sample is exposed to a suspension of test microbes and interfering substance and kept for specific contact times.
- After contact time, the test mixture is transferred to a neutralizing solution.
- Neutralizing extract is plated and incubated to enumerate remaining viable cells.
- Ensures that disinfectants are potent against mycobacteria, significantly lowering the risk of cross-infections within hospital environments.
- EN 14348 evaluates products in simulated practical conditions, ensuring reliable efficacy.
Turnaround Time
- Mycobacterium avium: 4–6 weeks
- Mycobacterium terrae: 3–4 weeks
The disinfectant’s performance is determined by calculating the log reduction in bacterial colonies.
Passing criteria
The disinfectant must achieve a 4-log reduction in viable mycobacteria under specified test conditions.
Do you have a product that needs testing?
Abstract
EN 14348 specifies a test method that determines the mycobactericidal (or tuberculocidal) activity of chemical disinfectant products intended for use in the medical area.
Mycobacterial strains show resistance to disinfectants because of their complex cell wall structure. Such strains are a more common cause of cross-infections and are associated with severe complications in hospital settings. Disinfectants claiming to possess tuberculocidal and mycobactericidal properties are tested against objectionable strains to determine their efficacy.
EN 14348 Test Conditions
- Mandatory test microorganisms –
For Mycobactericidal activity – Mycobacterium avium and Mycobacterium terrae
For Tuberculocidal Activity – Mycobacterium terrae
- Test temperature – 20 °C
- Contact time – 60 min
- Log reduction – Tested product must achieve 4 log reduction value
- Interfering substance
- Clean conditions – 0.3 g/l bovine albumin solution
- Dirty conditions – 3.0 g/l bovine albumin solution and 3.0 ml/l erythrocyte
EN 14348 Test Requirement
- Log reduction – The tested product must achieve a 4-log reduction value
- Product concentration – Products must be tested only at a concentration less than or equal to 80% as some dilution will always occur while adding the test organisms and interfering substances.
- Additional specific mycobacterial/tuberculocidal activity can be determined by applying other contact times, temperatures and interfering substances to meet the manufacturer’s intended claims.
EN 14348 Test Method
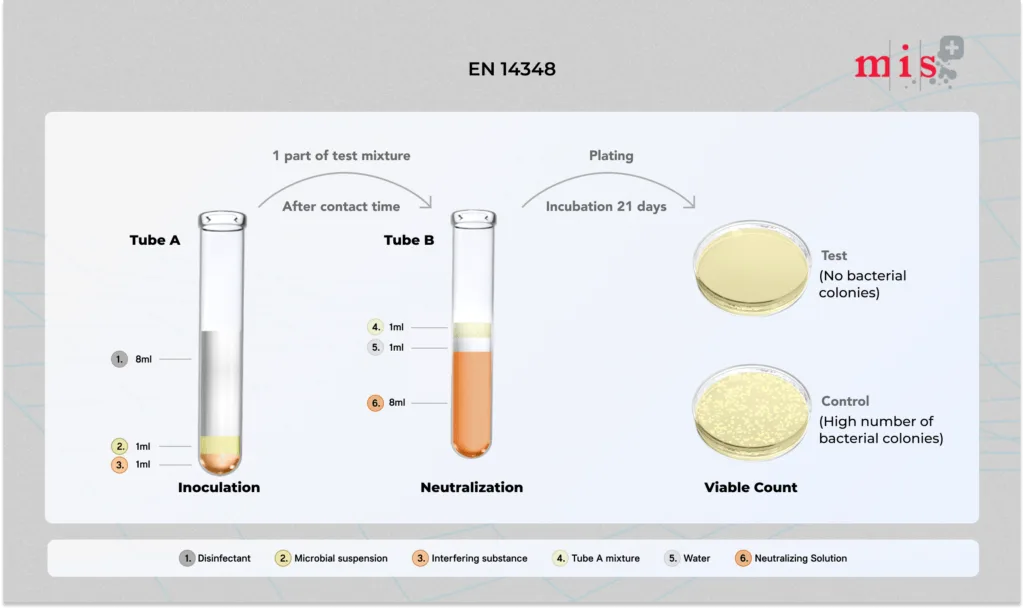
- Test sample is added into solution of test microorganisms and interfering substance. Mixture is allowed to interact for a specific duration.
- At the end of contact period, 1 ml of solution is further transferred into neutralizing solution to suppress bactericidal activity.
- Neutralizing extract is plated and incubated to enumerate remaining viable cells.
- After incubation, bacterial colonies are enumerated and results are compared to the control sample.
Log reduction
The tested products must demonstrate at least a 4-log reduction in the mycobacterial count to pass the test.
Applications of EN 14348 Test Method
The standard is applicable to disinfectant products that claim mycobactericidal/ tuberculocidal efficacy used in numerous settings, where disinfection is medically necessitated. These areas include but are not limited to:
- Healthcare Facilities: Hospitals, clinics, and dental institutions.
- Public and Community Spaces: Schools, nursing homes, and workplaces.
- Medical Devices: Instrument disinfectants and products regulated under the EU Medical Devices Directive.
- Other Areas: Laundry disinfectants and those used in kitchens that supply products directly to patients.
Benefits of BS EN 14348 Test method
- Compliance with regulatory requirements: Ensures adherence to European directives and medical regulations.
- Enhanced product credibility: Ensures that disinfectants are potent against mycobacteria, significantly lowering the risk of cross-infections within hospital environments.
- Simulates real-world conditions: The test evaluates disinfectants under conditions mimicking practical use, including varying organic loads and contact times.
- Reliable benchmarking: Provides a robust and standardized evaluation framework for disinfectants.
Conclusion
EN 14348 ensures that products meet stringent efficacy requirements, safeguarding public health in critical settings. By adhering to this standard, manufacturers can provide reliable and effective solutions for controlling mycobacteria.
MIS specializes in conducting EN 14348 disinfectant efficacy testing, ensuring compliance with the rigorous requirements of international standards.
With cutting-edge technology and a team of highly experienced experts, we deliver quality services with accuracy. Our commitment to excellence ensures that we meet our client’s project requirements efficiently and in a timely manner.
Additionally, our capabilities extend to performing EN 14563 disinfectant efficacy tests.
For more queries related to our microbiology testing services, talk to our experts here.
Frequently Asked Questions

DR. Martinoz Scholtz
EN 14348 test is phase 2 step 1 quantitative suspension test that determines mycobactericidal activity of chemical disinfectants intended for use in medical areas.
EN 14348 test is applicable for chemical disinfectants and instrument disinfectants to be used in areas or situations where disinfection is medically indicated.
Turnaround time for testing against Mycobacterium avium will be four to six weeks. While
testing against Mycobacterium terrae will take three to four weeks.
At Microbe Investigations Switzerland, we test for EN 14348 using the following microbial strains: Mycobacterium avium and Mycobacterium terrae.
No, disinfectant wipes cannot be tested under EN 14348 as this is a suspension test, not a carrier test.
While EN 14348 is highly effective in assessing mycobactericidal activity, its scope is limited to disinfectants intended for medical areas and specific test organisms.
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
Antibacterial testing of disinfectants plays
Antimicrobial testing is important to
Antibacterial efficacy testing is an
Let’s face it, we are