- Swiss testing laboratory
BS EN 14347:2005

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Related tests for you
Quick understanding of the test
BS EN 14347:2005 - Evaluation of Basic Sporicidal activity of Chemical Disinfectants and antiseptics (phase 1, step 1)
Application
- Bacillus Subtilis (ATCC 6633)
- Bacillus Cereus (ATCC 12826)
- A bacterial spore suspension is mixed with the disinfectant sample and allowed to interact for a specified contact time.
- After the contact time, an aliquot is transferred to a neutralizing solution.plated, and incubated.
- Neutralized extract is then plated and incubated to enumerate surviving bacteria and calculate the reduction rate.
- Provide a preliminary step to screen the effectiveness of disinfectants against spores under standardized conditions before moving to more complex and specific testing phases.
- Helps manufacturers ensure that their products meet minimum requirements for sporicidal activity.
Turnaround Time
Passing criteria
Do you have a product that needs testing?
Abstract
EN 14347 is phase 1, step 1 quantitative suspension to test the basic sporicidal activity of disinfectant formulations in the early stages of development.
This standard is applicable for products intended to be used in the areas/situations such as hospitals, veterinary areas, agricultural (no crop protection), institutional, domestic services, food hygiene and industrial settings.
EN 14347 Test Conditions & Requirement
- Mandatory test microorganisms – Bacillus Subtilis (ATCC 6633) and Bacillus Cereus (ATCC 12826)
- Test temperature – 20 °C
- Contact time – 30 mints, 60 mints, 120 mints
- Passing criteria – Products must demonstrate ≥ 4 log reduction in comparison to control sample
EN 14347 Test Method
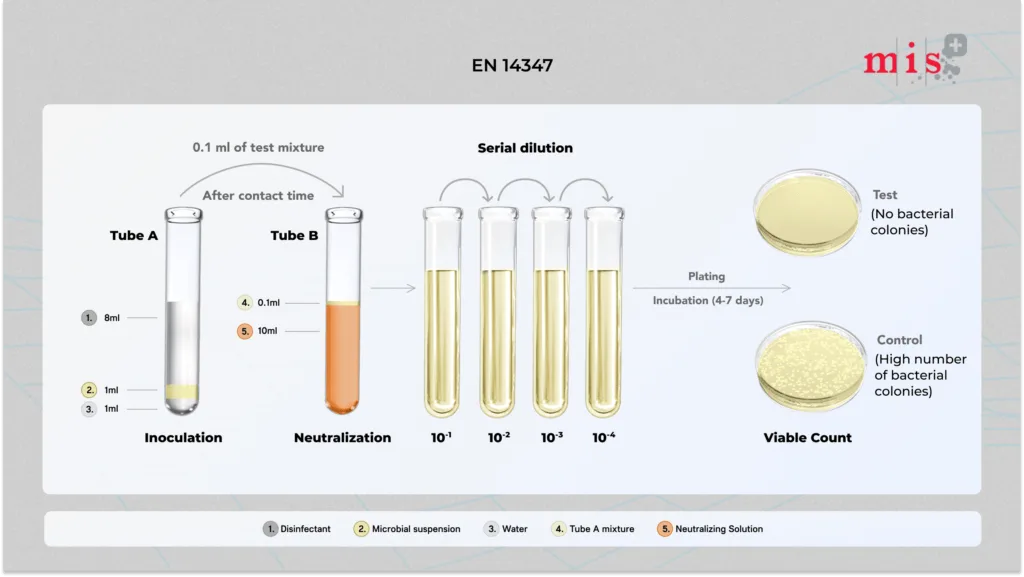
- A test suspension of bacterial spores is added to the disinfectant sample and allowed to interact at 20 °C for a specified contact time.
- After the contact time, an aliquot of the mixture is transferred into a neutralizing solution to neutralize sporicidal activity.
- Then neutralizing extract is plated and incubated for 4-7 days to allow proliferation of any remaining spores.
- After incubation, viable cells are counted and results are compared to control samples.
Importance of EN 14347 Test
In Europe EN 14347 Test, phase 1, step 1 suspension test is a fundamental part of the registration process for disinfectants. This test is performed during the developmental stage of the product to demonstrate whether active ingredients used in product formulation possess their respective antimicrobial properties against bacteria, viruses, fungi, viruses.
Thereby, it is a prerequisite for manufacturers of chemical disinfectants and antiseptic products to meet all efficacy requirements for basic suspension (phase 1) as outlined in EN guidelines. EN 14347 test is designed to harmonise the manufacturing and performance of disinfectant formulations that are planned to be used in medical, veterinary, food, industrial, domestic and institutional areas.
Conclusion
Need help with EN 14347 testing of your product samples, MIS team experts would be happy to serve you. We are a team of microbiology experts and specialists who are constantly betrothed in understanding customer needs and offering right solutions.
Through technical competence of our testing facilities, we also carry out phase 2 step 1, phase 2 step 2 tests for disinfectant/ antiseptic formulations.
Along with EN 14347 (phase 1) testing, EN 14348 (phase 2, step 1) is the most frequently requested and performed test method at MIS.
For more detailed information about our disinfectant efficacy testing, contact our experts today.
Frequently Asked Questions

DR. Martinoz Scholtz
EN 14347 test is a basic quantitative suspension test for chemical disinfectants and antiseptics. To meet EN passing criteria, test product must demonstrate sporicidal efficacy against two obligatory microorganisms – Bacillus Subtilis and Bacillus Cereus.
EN 14347 test is applicable to chemical disinfectants and antiseptics which have sporicidal activity.
EN 14347 test takes 2-3 weeks to complete.
At Microbe Investigations, we test for EN 14347 using the following strains Bacillus Subtilis (ATCC 6633) and Bacillus Cereus (ATCC 12826). Additional strains can be added on client’s request.
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
Did you know there are
Antibacterial testing of disinfectants plays
Antimicrobial testing is important to
Antibacterial efficacy testing is an
Let’s face it, we are