Home / Disinfectant Testing / antiviral / ASTM E1838
- Swiss testing laboratory
ASTM E1838 : 2017

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Related tests for you
Quick understanding of the test
ASTM E1838 : 2017 - Evaluating the virus-eliminating efficacy of hygienic handwash and handrub agents.
Application
- Murine Norovirus Type 1 (TIB-71)
- Feline Calicivirus (CCL-94).
- A known volume of virus suspension is applied to the fingerpad and allowed to dry.
- The contaminated area is then exposed to the test agent for a predetermined time.
- The remaining virus on the fingerpad is eluted, titrated, and compared to a control to determine the viral reduction.
- Provides a consistent methodology for testing the effectiveness of handwash and handrub products.
- Delivers quantitative data on the percent reduction of virus, allowing for clear comparisons between different products and formulations.
Turnaround Time
Passing criteria
Do you have a product that needs testing?
Abstract
ASTM E1838 is a standardized in vivo test method developed to assess the virus-eliminating effectiveness of hygienic handwash and handrub agents using the fingerpads of adult volunteers. Since human skin does not naturally harbor resident viral flora, the method focuses on measuring how well these products reduce transient viral contamination either by inactivation or removal. The procedure simulates real-world contamination and hand hygiene scenarios, ensuring that products are tested under conditions representative of actual use.
Scope of products for testing
This method is specifically applicable to:
- Liquid hygienic handwash and Handrub agents intended for use in healthcare facilities, daycare centers, nursing homes, and food-handling settings.
Excluded Products:
- Surgical hand scrubs.
- Preoperative skin preparation agents.
ASTM E1838 Test Conditions & Requirement
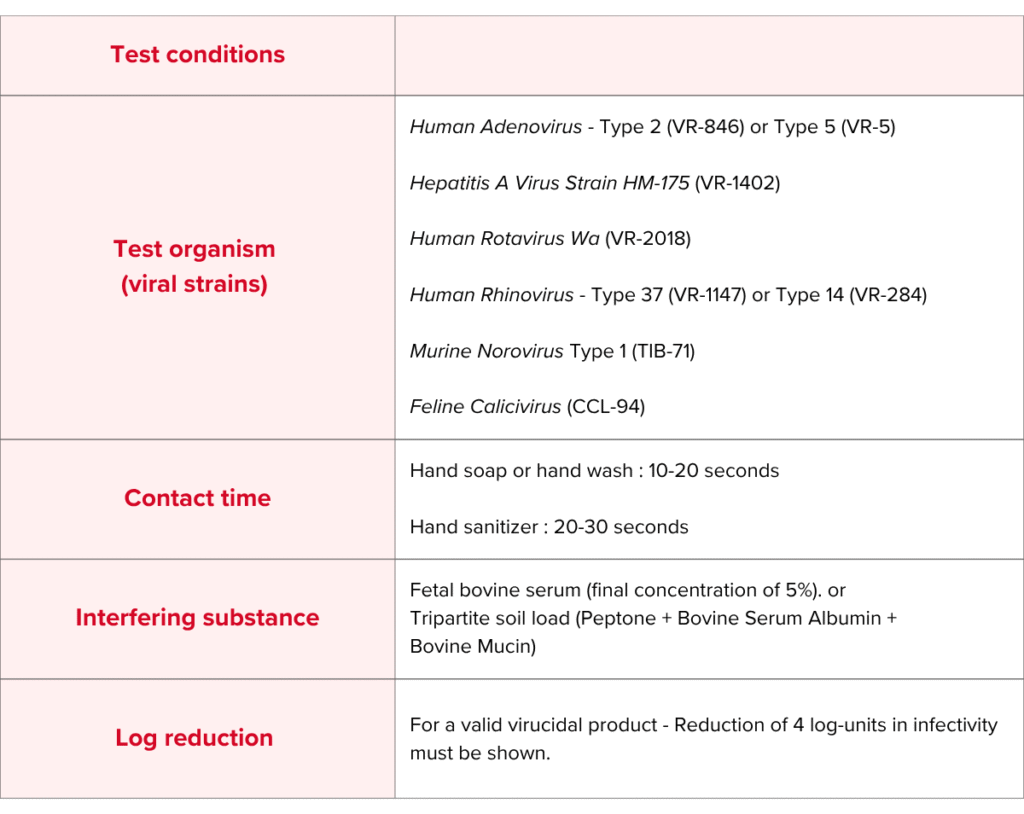
ASTM E1838 Test Method
- ASTM E1838 test is performed on the finger pad of healthy volunteers with undamaged skin.
- Volunteers’ hands are washed with non germicidal soap for 10 seconds and dried out. Further, the hands are precleaned by 70% ethanol and rubbed against each other until they are dry.
- The defined area of finger pads are contaminated by viral inoculum and allowed to visibly dry for 20 to 25 minutes.
- At the end of drying, any of the two fingerpads are eluted in order to measure “Baseline control”.
- Dried inoculum on other fingerpads are exposed to 1ml of test product or control fluid for desired contact time.
- To determine the virus elimination after exposure to test product, fingerpads can be eluted without additional treatment.
- Fingerpads exposed to test product are further rinsed with 1ml of hard water for 5-10 seconds.
- Virus can be eluted from fingerpads at this stage or after drying of hands.
- The eluates collected and controls are titrated for virus and the log reduction value is calculated.
- After the test, the hands of volunteers are decontaminated and dried completely before leaving the test area.
Importance of ASTM E1838 Test
Infection control begins with clean hands. Viruses, unlike bacteria, do not naturally reside on human skin, but contaminated hands are a major vehicle for viral transmission especially in environments like hospitals, daycare centers, and food service settings. Many common viruses can survive on the hands for hours and are more resilient than typical bacterial test organisms. ASTM E1838 provides a robust and scientifically validated method to evaluate whether hand hygiene products truly reduce viral loads. This test plays a critical role in product development and regulatory claims, allowing manufacturers to substantiate antiviral efficacy through a standardized protocol.
Conclusion
At MIS, all the antiviral, antibacterial and antifungal testing services are carried out in accordance with international standards and guidelines. With the help of our qualified staff and certified advanced laboratory set up, we ensure to provide precise, reliable and expeditious services to our clients.
To know more about ASTM E1838 testing, turnaround time and any other related queries contact us here.
Frequently Asked Questions

DR. Martinoz Scholtz
ASTM E1838 specifies a test method to evaluate the effectiveness of handwash and handrub formulations for inactivating and/or removing transient viruses from the hands.
ASTM E1838 test can be carried out for microbicidal and non microbicidal hand wash or handrub products that claim to kill/eliminate viral pathogens.
ASTM E1838 test takes 5-6 weeks to complete.
At MIS, we test for ASTM E1838 using the following viral strains : Human Adenovirus – Type 2 (VR-846) or Type 5 (VR-5), Hepatitis A Virus Strain HM-175 (VR-1402), Human Rotavirus Wa (VR-2018), Human Rhinovirus – Type 37 (VR-1147) or Type 14 (VR-284), Murine Norovirus Type 1 (TIB-71) and Feline Calicivirus (CCL-94).
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
The global threat from viral
The health threats posed by
Let’s face it, we are