The CSN EN 1040 standard sets requirements for testing of the bactericidal activity of chemical disinfectants and antiseptics. An understanding of the standards by the manufacturers is important for ensuring the ability of the product to meet European efficacy requirements for disinfection practices. This article is a guide for the implementation of EN 1040 standards into manufacturing practices to ensure product effectiveness against bacteria under specified conditions.
What is the EN 1040 Standard?
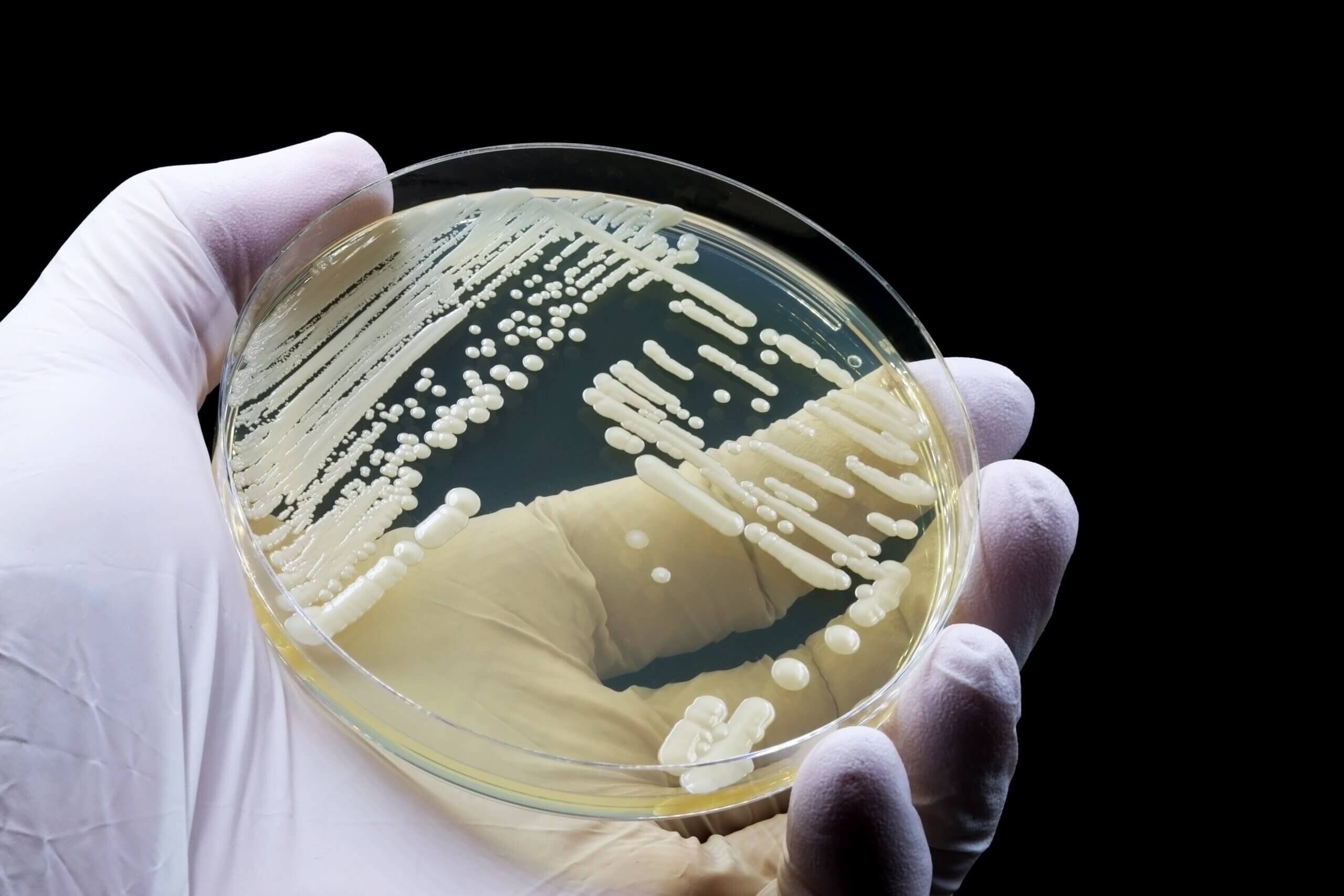
EN 1040 is a quantitative suspension test to evaluate the basic bactericidal activity of chemical disinfectants and antiseptics. It measures how a product kills bacterial cells under laboratory controlled conditions and can be seen as a preliminary screening test for disinfectants.
Key Components of standard
- Test Organisms: Pseudomonas aeruginosa and Staphylococcus aureus are the mandatory test organisms.
- Concentration: Disinfectants are tested at various concentrations starting from 80% concentration by diluting with water.
- Contact Time and Temperature: Standard conditions include a contact time of 5 minutes at a temperature of 20°C.
- Reduction Requirement: A successful test shows a minimum of 5-log reduction in the bacterial count.
Introducing EN 1040 in Manufacturing Processes
The implementation of the EN 1040 standards in the product manufacture requires a number of well-structured steps from the selection of active ingredients to the product testing.
1. Component Compliance
Quality Assurance
- Make sure that all active constituents are having bactericidal properties according to the requirements of EN 1040 tests.
- Ensure that the supplier’s certificates of compliance for the raw materials are applicable to all batches.
2. Formulation Development
Optimizing Bactericidal Concentrations
- Formulations are developed in such a way that they maintain their bactericidal efficacy when diluted to 80% or less, as stipulated by the EN 1040 test standard.
- Preliminary in-house testing is conducted to check the bactericidal activity using the specified strains of bacteria.
3. Process Adaptation and Control
Equipment and Calibration
- Employ equipment for proper mixing and storage that avoids contamination and any degradation of bactericidal properties.
- The equipment must be regularly calibrated for consistent product quality based on the EN 1040 test standard.
Batch Processing
- Install continuous monitoring systems to maintain necessary levels of concentration and pH while still supporting bactericidal properties.
4. Quality Control and Validation
In-House Testing
- In-house microbiology labs to conduct EN 1040 tests should be established to ensure that each production batch meets the bactericidal activity standards before release.
- Use validated neutralizers to ensure that the bactericidal activity is accurately measured without interference from the disinfectant chemicals.
Third-Party Certification
- Regularly schedule third-party testing to validate in-house test results and maintain certification compliance.
- Certified reference materials and standardized procedures should be used to confirm reliability and repeatability of test results.
5. Documentation and Regulatory compliance
Keeping records
- Detailed records of each batch tested, including test organism strains, batch numbers, test results, and conditions should be maintained.
- All quality checks and calibrations of equipment used in the manufacturing process are documented.
Regulatory Compliance
- Regularly review and update processes to comply with the latest EN standards such as EN 1040 test and other relevant regulations.
- Prepare comprehensive documentation for regulatory inspections and audits.
Challenges and Solutions
Implementation of EN 1040 test in the Manufacturing sector is possible despite the challenges like variability in the quality of raw materials and changes in the regulatory environment. These challenges can be managed with robust quality control systems, periodic staff training on compliance and testing procedures, and adaptive process management to accommodate legislation’s new requirements.
Meeting the EN 1040 test standards is a stringent requirement that should be met in order to ensure that a high quality of disinfectant is produced. There needs to be a systematic way of integrating these standards into each stage of the manufacturing process, from raw material selection to final product testing, so that manufacturers can be assured of both efficacy and competitiveness in markets demanding high standards of public health protection
At Microbe Investigations Switzerland (MIS), we understand the critical importance of adhering to standards like EN 1040 to ensure your disinfectant products are effective and compliant. With our state-of-the-art laboratory, equipped with the latest technology, we offer comprehensive testing services designed to meet your needs. Our team of experienced experts is committed to providing you with accurate, reliable, and timely testing services. Contact us today to learn more about our tailored testing service and get a quotation for the EN 1040 test.