- Swiss testing laboratory
BS EN 17914: 2022

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Related tests for you
Evaluation of Virucidal Activity of Chemical Disinfectants on Hard Non-Porous Surfaces
Quick understanding of the test
BS EN 17914: 2022 - Quantitative suspension test method for evaluating the virucidal activity of chemical disinfectants and antiseptics in food, industrial, domestic, and institutional areas
Application
- Adenovirus
- Murine Norovirus
- Vaccinia Virus
- Poliovirus
- Parvovirus
- The test sample with the interfering substance is challenged with the test virus suspension and kept for incubation.
- After the contact time, the test mixture is neutralized. Neutralized extract is then serially diluted and transferred to a 96-well plate, and incubated.
- After the incubation period, the number of infective viruses is calculated using the TCID50 method.
- EN 17914 standard ensures that products used in critical environments such as food handling and healthcare are effective against viruses, thereby supporting public health and safety measures.
- Compliance with this standard helps manufacturers meet regulatory requirements, contributing to product approvals and market trust.
Turnaround Time
Passing criteria
Do you have a product that needs testing?
Abstract
BS EN 17914: 2022 is a phase-2 step-1 quantitative suspension test method that lays out a guideline for assessing the virucidal activity of a given chemical disinfectant or antiseptic intended to be used in the
- Food
- Industrial
- Domestic
- Institutional areas.
The test parameters have been formulated to closely mimic the practical application condition for the disinfectant. The standard is applicable to a test product that forms a homogeneous, physically stable preparation when diluted with hard water – or in the case of ready-to-use products, i.e., products that are not diluted when applied – with water.
EN 17914 Test Conditions & Requirements:
Test Conditions | Hygienic handrub and handwash | Instrument disinfection | Surface disinfection | Textile disinfection |
Mandatory test organisms | Non – enveloped viruses | |||
Poliovirus Adenovirus Murine Norovirus | Poliovirus Adenovirus Murine Norovirus when temperature is 40 °C or higher, only Parvovirus | Poliovirus Adenovirus Murine Norovirus | Parvovirus | |
Limited spectrum virucidal activity Adenovirus Murine Norovirus | -NA – | Limited spectrum virucidal activity Adenovirus Murine norovirus | -NA – | |
Enveloped viruses | ||||
Vaccinia virus | -NA – |
vaccinia virus | -NA – | |
Additional | Any relevant test organism | |||
Test temperature | According to the manufacturer’s recommendation, but at/between | |||
20 °C | 20 °C and 70 °C | 4 °C and 30 °C | 30°C and 70 °C | |
Contact time | According to the manufacturer’s recommendation | |||
between 30 s and 120 s | But no longer than | |||
60 min | 5 min or 60 min | 20 min | ||
Interfering substance – clean conditions | 0,3 g/l bovine albumin solution (hygienic handrub) | 0,3 g/l bovine albumin solution | 0,3 g/l bovine albumin solution | 3,0 g/l bovine albumin solution plus 3,0 ml/l erythrocytes |
Interfering substance – dirty conditions | 3,0 g/l bovine albumin solution plus 3,0 ml/l erythrocytes (hygienic handwash) | 3,0 g/l bovine albumin solution plus 3,0 ml/l erythrocytes | 3,0 g/l bovine albumin solution plus 3,0 ml/l erythrocytes | 3,0 g/l bovine albumin solution plus 3,0 ml/l erythrocytes |
EN 17914 Test Method
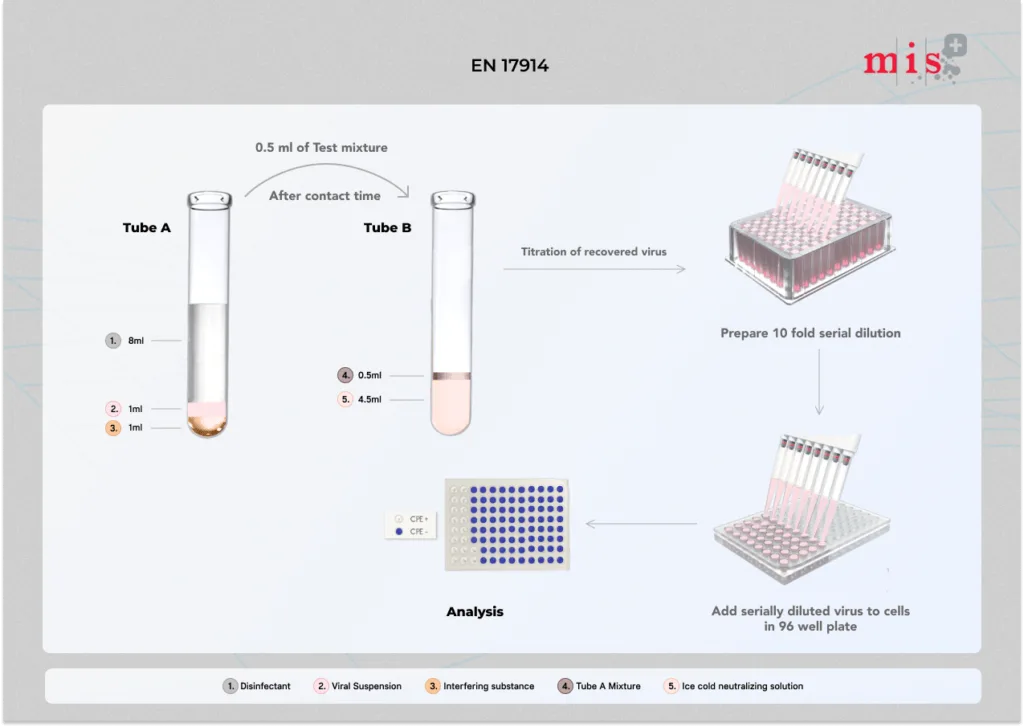
- The given test sample (disinfectant) or hard water is first mixed with an interfering substance, followed by challenging the mixture with the test virus.
- The contact time for which the disinfectant sample is challenged and the temperature at which the mixture is incubated is as per the manufacturer’s recommendations or the standard guidelines.
- At the end of the contact time, an aliquot (small portion) of the test mixture is taken, and the antiviral action of the disinfectant sample is terminated using a neutralizing medium.
- The neutralizing medium with the virus is serially diluted and transferred onto a permissive monolayer of cells in a 96-well plate.
- The 96 well plates are incubated in a CO2 incubator for a specific period of time depending on the test virus.
- After the incubation period, 96 well plates are examined for CPE (cytopathic effect), and the number of the infective virus is calculated using the TCID50 method.
- Antiviral efficacy of the disinfectant sample is measured by comparing the virus titer with the control (without test product) sample.
Importance of EN 17914 Test
EN 17914 guideline helps in evaluating the efficacy of disinfectant/antiseptic products in preventing the transmission and spread of any viral disease in the
- Food
- Industrial
- Domestic
- Institutional areas.
The disinfectant sample passing the EN 17914 test will help to maintain a safe and healthy environment. EN 17914 also serves as the standard testing guideline for registering and selling the disinfectant sample in the European zone and some other countries.
Why choose MIS Lab to conduct EN 17914 testing?
MIS performs routine virucidal tests for disinfectants implied to be used in different areas of applications.
Our state-of-the-art testing facility is one of only a few facilities worldwide that provides efficacy research and data for products with antiviral/ virucidal potential.
Additionally, EN 14476, EN 17111, ASTM E 1053, and EN 16777 tests are also available for disinfectant virucidal claims in the MIS lab.
To get a quote on the EN 17914 test or if you need any additional information regarding our testing services, please contact our experts today.
Frequently Asked Questions

DR. Martinoz Scholtz
EN 17914 is a phase-2 step-1 test that specifies a suspension test method for evaluating the virucidal activity of chemical disinfectants and antiseptics used in the Food, Industrial, Domestic, and Institutional areas.
EN 17914 test applies to disinfectant/antiseptic products intended for disinfecting without mechanical action on non-porous surfaces in the Food, Industrial, Domestic, and institutional areas.
EN 17914 test takes 5-6 weeks to complete.
At Microbe Investigations, we test for EN 17914 using the following microbial strains: Adenovirus, Poliovirus, and Murine norovirus for non-enveloped viruses and Vaccinia Virus for enveloped viruses.
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
The re-emergence of monkeypox, a
The global threat from viral
The health threats posed by
Let’s face it, we are