- Swiss testing laboratory
ISO 18184: 2025 test method

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Summary
ISO 18184 is an international test standard that helps assess the antiviral activity of textile products by measuring the reduction of infectious virus on treated fabrics. Applicable to woven, knitted, and nonwoven textiles, including face masks, medical textiles, and functional fabrics, with test results reported as log reduction values. ISO 18184 supports antiviral performance claims and helps manufacturers demonstrate efficacy for protective and functional textile applications.
Related tests for you
Quick understanding of the test
ISO 18184 test method - This International Standard (ISO 18184: 2025) specifies testing method for the determination of the antiviral activity of the textile products against viruses.
Applicable for a wide range of textiles including woven and knitted fabrics, non-woven fabrics, cotton, fibres, yarns, braids, feathers,surgical clothes, masks or similar products.
Mandatory test strains
- SARS-CoV-2
- Influenza A (H1N1, H3N2)
- Human Coronavirus (229E)
- Beta Coronavirus (OC43)
- Samples and controls are challenged with the test virus.
- After incubation, samples are neutralized with a suitable neutralizing solution.
- Virus recovered in the neutralizing solution is then serially diluted, and the infectious titre of the recovered virus is determined using plaque assays or TCID50 assays.
Benefits
- Test assesses both enveloped and non-enveloped viral strains, thus providing a comprehensive product assessment.
- Provides measurable data on antiviral activity (log reduction), offering actionable insights for manufacturers.
Turnaround Time
- Standard turnaround: 4 weeks.
- Fast Track program: 2–3 weeks (based on the virus strain).
Results
Passing criteria
Do you have a product that needs testing?
What Is ISO 18184 Test?
ISO 18184, originally published in 2014 and revised in 2019, is a standardized test method for evaluating the antiviral activity of textile products. The 2025 update expands its scope to cover both woven and non-woven materials. ISO 18184:2025 provides a reliable framework for manufacturers and laboratories to evaluate and ensure textile efficacy, supporting the growing need for antiviral protection in textiles due to rising viral threats.
ISO 18184 is applicable when quantitative antiviral efficacy data is required to support regulatory submissions or substantiated antiviral performance claims.
Textile Products Applicable for ISO 18184
ISO 18184 can be used to evaluate the antiviral activity of different textile products, such as
- woven, non-woven & knitted fabrics,
- yarns,
- active wear,
- socks
- daily wear,
- health care products such as scrubs, masks, & surgical clothes,
- and other home textiles.
ISO 18184 is used for textile products that are hydrophilic in nature. For hydrophobic textiles, ISO 21702 shall be used to evaluate antiviral activity.
ISO 18184 is also a commonly used method for evaluating the efficacy of antiviral face masks.
Know here – Face Mask Antiviral Testing standards: ISO 18184 vs. JIS L 1922
Mandatory Virus Strains in ISO 18184
- Influenza A H3N2
- Influenza A H1N1
- Feline calcivirus
Sample Preparation Requirements
Control Sample – 9 sterilized control (untreated) specimens with a mass of 0.4g ± 0.05g are required for ISO 18184 testing.
- 3 control specimens are used to determine the infectious titre of the virus immediately after inoculation.
- 3 control specimens are used to determine the infectious titre of the remaining virus after inoculation for a “contact time” (Standard “contact time” is 2 hours, but can go up to 24 hours).
- 3 control specimens are used for cytotoxicity analysis.
Test samples – 6 sterilized test (treated) samples with a mass of 0.4g ± 0.05g are required for ISO 18184 testing.
- 3 treated specimens to determine the infectious titre of the remaining virus after inoculation is in contact with the treated specimen, same as the control specimen.
- 3 treated specimens are used for cytotoxicity analysis.
We test using following organisms: SARS CoV-2, Beta Coronavirus (OC-43) (ATCC VR-1558), Human Coronavirus (229E) (ATCC VR-740), Influenza A (H1N1) (ATCC VR-1469), Influenza A (H3N2) (ATCC VR-1679), Adenovirus, Norovirus, Poliovirus, Vaccinia virus, Feline Calicivirus
ISO 18184 Test Procedure
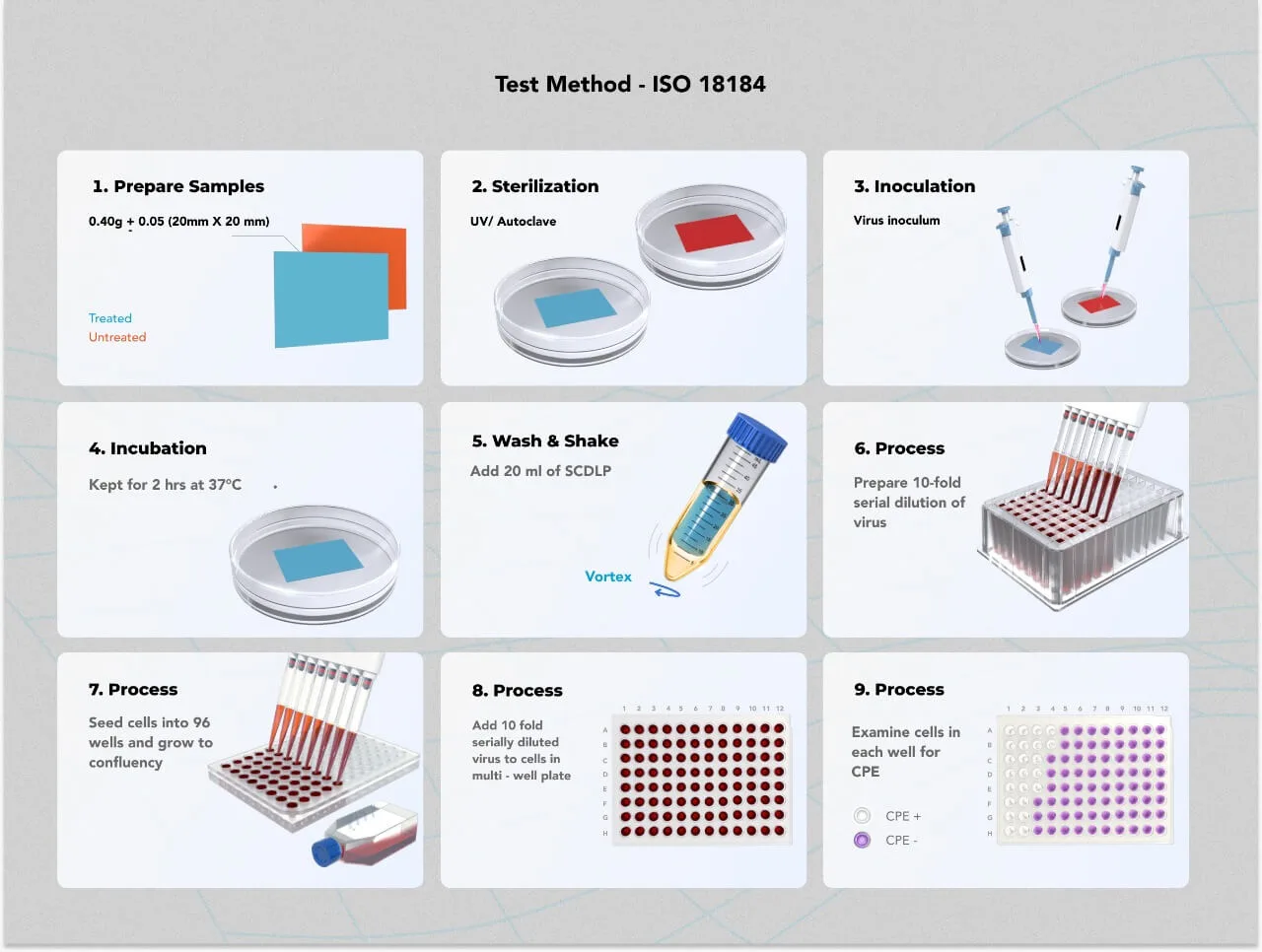
- Control and treated samples are placed in separate sterile plates.
- The 200 uL of the virus is inoculated on both the control and the treated samples.
- Immediately after the inoculation of the virus, 20 ml of SCDLP ( used as a neutralizing solution) is added to 3 control samples.
- After a specific contact period, 20 ml of SCDLP broth is added to 3 treated and 3 untreated samples to recover the remaining virus.
- The wash-out solution is serially diluted up to 10 dilutions, and the infectious titre of the recovered virus is determined either by Plaque assay or by TCID50 assay. Other assays can also be used based on the virus strain.
Antiviral Activity Calculation & Log Reduction
Antiviral Activity and Performance Standard
The antiviral activity is determined by the following equation.
Mv = Log10 (Va) – Log10 (Vc)
Where,
Mv is the antiviral activity value
Log10 (Va) is the logarithm average of 3 infectivity titre value immediately after inoculation of the control specimen
Log10 (Vc) is the logarithm average of 3 infectivity titre value after specific contact time with the test specimen.
As per the ISO 18184, If the log value is between 2 and 3, the antiviral performance of the textile product is considered good. If the log value is greater than or equal to 3, then the antiviral performance of textile product is considered excellent.
Antiviral activity value (Mv) | Efficacy Rating |
2 ≤ Mv < 3 | Good Effect Level |
Mv ≥ 3 | Excellent Effect Level |
Table 1: Antiviral performance standard
ISO 18184 vs Other Standards
Parameter | ISO 18184 | ISO 21702 | JIS L 1922 |
Purpose | To determine antiviral activity of textiles (porous surfaces) | To determine antiviral activity on plastics and other non-porous surfaces | To determine antiviral activity of textiles (porous surfaces) – adopted from ISO 18184 |
Test organisms | Influenza A H3N2 Influenza A H1N1 Feline calcivirus | Influenza A H3N2 Feline calcivirus | Influenza A H3N2 Influenza A H1N1 Feline calcivirus |
Scope of products | Woven and knitted fabrics, fibers, yarns, nonwovens, and other textile materials | Plastics, coatings, ceramics, rubber, leather, stainless steel, and other non-porous materials | Woven and knitted fabrics, fibers, yarns, braids, and other textile products |
Results interpretation | Quantitative log reduction – ≥2 log = good effect ≥3 log = excellent effect (commonly used guidance) | Quantitative log reduction comparison between treated vs control surfaces | Quantitative log reduction comparison between treated vs control textiles |
Regulatory body | International Organization for Standardization (ISO) | International Organization for Standardization (ISO) | Japanese Industrial Standards (JIS), based on ISO 18184 |
Why Choose Our Lab for ISO 18184 Testing
At Microbe Investigations Switzerland (MIS), we specialize in evaluating the antiviral performance of treated textiles under ISO 18184, generating data you can confidently use for ensuring performance claims.
We test woven, non-woven, and knitted fabrics as well as finished textile products using ISO 18184 and JIS L 1922, ensuring reliable and reproducible antiviral efficacy results.
Beyond textiles, we also offer a comprehensive array of testing services to determine the antimicrobial efficacy of plastics, coatings, and surface disinfectants.
Request ISO 18184 Testing
Looking to validate the antiviral performance of your textile products in accordance with ISO 18184? Contact our experts
Frequently Asked Questions

DR. Martinoz Scholtz
Yes, ISO 18184 can be used to measure antiviral activity of face mask material but it does not assess filtration efficiency or breathability performance.
ISO 18184 tests antiviral activity on textiles/porous materials, whereas ISO 21702 measures antiviral activity on plastics and other non‑porous surfaces.
Yes, ISO 18184 can be used to support antiviral marketing claims.
The test standard lists down Influenza A H3N2, Influenza A H1N1 and Feline calicivirus as mandatory viral strains.
Yes. Nonwoven textile materials used in PPE can be tested.
Antiviral activity Value (Mv) between 2 log ≤ Mv < 3 log reduction is considered good and ≥3‑log (≥99.9 %) reduction as excellent antiviral activity according to the standard.
The turnaround time for the ISO 18184 test is 4-5 weeks.
Yes, ISO 18184 can be used to check the antiviral performance of nonwoven textile materials.
Antiviral activity in ISO 18184 is calculated as the logarithmic reduction in viral titer recovered from the treated textile compared to the untreated control after the defined contact time.
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
Face masks coated with antibacterial
Introduction The Japanese Industrial Standards