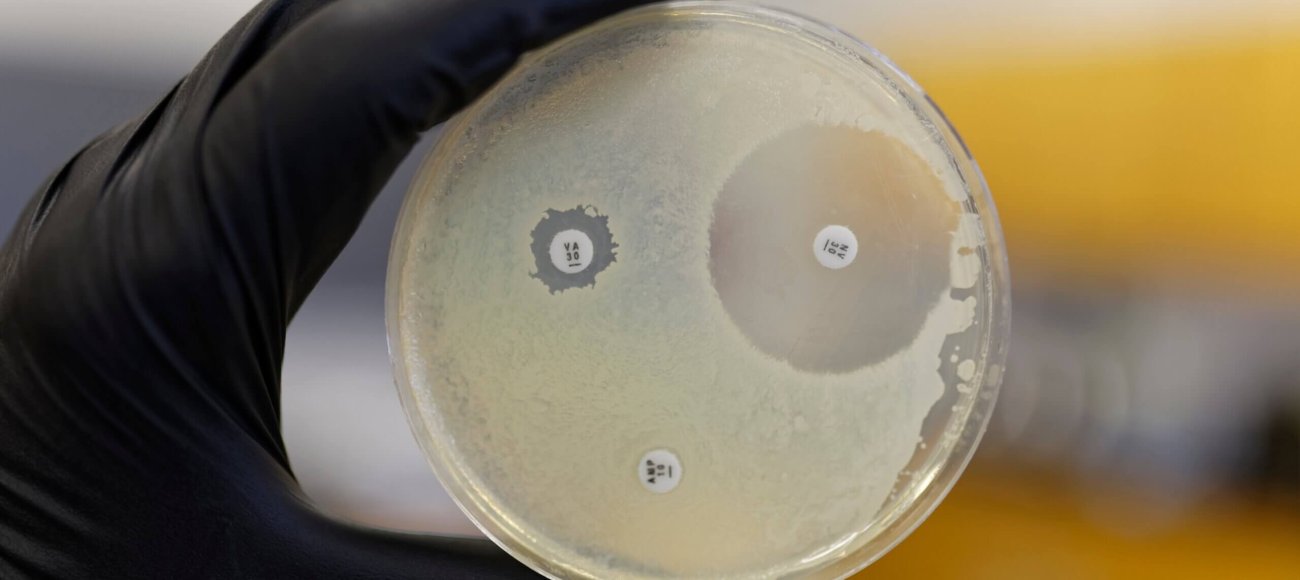
Zone of Inhibition (ZOI) testing is a fundamental method used in microbiology to assess the effectiveness of antimicrobial agents. This test measures the area around a treated sample where bacterial growth is inhibited, providing a clear indication of the antimicrobial properties of the substance being tested. The larger the zone, the more effective the antimicrobial agent is considered to be.
Importance of ZOI Testing
ZOI testing is crucial in various fields, including pharmaceuticals, healthcare, and agriculture, where it helps in identifying substances that can control or eliminate harmful microorganisms. This testing method is essential for developing new antibiotics, disinfectants, and antiseptics. By determining the effectiveness of these agents, ZOI testing plays a significant role in ensuring public health and safety.
Factors Influencing ZOI Testing
While the ZOI test is straightforward, several factors can influence the results, including the concentration of the antimicrobial agent, the type of microorganism, and the incubation time. However, one of the most critical yet often overlooked aspects is the environmental conditions under which the test is conducted.
The Importance of Environmental Factors in ZOI Testing Results
1. Temperature
Temperature is a vital environmental factor that can significantly affect ZOI testing results. Temperature determines the rate at which the bacteria grow and the rate at which the antimicrobial agent diffuses through the agar medium. In normal circumstances, ZOI testing is carried out at 35-37°C, which is optimum growth temperature for most pathogenic bacteria.
1.1 Effect of Temperature on Bacterial Growth
Even minor changes in temperature might lead to slight deviations in bacterial growth rates. If the incubation temperature is too low, the rate of bacterial growth may be slow or the bacteria might not grow at all, and the effect will be the presence of larger zones of inhibition, which do not reflect the actual antimicrobial potency. If the incubation were to be higher, then bacterial growth would be accelerated, which could lead to smaller zones of inhibition and probably underestimate the antimicrobial activity.
1.2 Effect of Temperature on Antimicrobial Diffusion
In general, higher temperatures lead to diffusion rates that are higher, and ideally, ZOIs would be bigger under increased temperatures. Lower temperatures decrease the rates of diffusion and, by implication, the diameters of the zones of inhibition. For this reason, it is of paramount importance to keep the temperature within the incubator constant in order to ensure that the measurements of the ZOI are reproducible and correct.
2. pH of the Medium
The pH in the agar medium greatly impacts the activity of antimicrobial agents and bacterial growth. Most of the antimicrobials show optimal pH ranges at which their action is best, and changes from this range affect activity.
2.1 Optimal pH for Antimicrobial Agents
Each of the antimicrobial agents has a certain pH range within which maximum efficacy is achieved. Aminoglycosides, for instance, tend to show their maximum activity in alkaline conditions, while for tetracyclines, they are more active in slightly acidic conditions. Deviations from that optimal pH range might result in decreased efficacy of the antimicrobial agents, which in turn yields the wrong Zone of Inhibition (ZOI).
2.2 Impact on Bacterial Physiology
The growth rates and physiology of bacterial cells could also be affected by the pH of the medium. An extreme pH can inhibit the growth of bacteria or change the permeability of the bacterial cell membrane. The permeability of the cell membrane would further impact the interaction of the bacteria with the antimicrobial agent. Agar medium should have approximately neutral pH to do the ZOI test with reliability.
3. Agar Depth and Composition
Important parameters for predicting the results of the ZOI test are the chemical composition and important depths of the agar medium, as these impact the diffusion of the antimicrobial agent and the growth of bacteria.
3.1 Agar Depth
The normal depth for agar in ZOI testing is 4 mm. Incongruous results are developed via the depth variations. A thicker layer of the agar would act in a way that the diffusion of the antimicrobial agent through the medium would be retarded and hence a smaller inhibition zone. On the other hand, the thinner layers of the agar could help in the diffusion, thus having more considerable zones of inhibition. The agar depth uniformity in all the test plates has to be maintained to get reproducible results.
3.2 Agar Composition
It is also important for ZOI testing that the formulation be in terms of nutrients, the type and concentration of nutrients, and the gelling agents used to prepare the agar medium. The primary reason for employing Mueller-Hinton agar medium is to make it supportive for the growth of a broad spectrum of bacteria and consistent in the composition. Differences in the formulation or preparation of the agar will bring about differences in bacterial growth and antimicrobial diffusion, which would in turn affect the size of the inhibition zones.
4. Inoculum Density
Another variable is the density of the bacterial inoculum used in the ZOI test. In many cases, this is calibrated to the standard density of the inoculum, being the equivalent of a 0.5 McFarland standard, which corresponds, in turn, to approximately 1.5 x 10^8 CFU/mL.
4.1 Effect on Zone of Inhibition
Inoculum density directly affects the size of the inhibition zones. More inoculum density will support smaller zones of inhibition because there will be more bacteria to be killed by the antibacterial agent. On the contrary, lower inoculum density can really be associated with larger inhibition zones. So, proper inoculum density is very important for correct and comparable results.
4.2 Standardization Techniques
The inoculum density is standardized by adjusting the turbidity of bacterial suspensions by spectrophotometric analysis or by visual matching to a McFarland standard. Standardizing the inoculum density would give constant results for every test and therefore less variation thus improving the results of ZOI.
5. Humidity and Incubation Conditions
Other factors relevant to ZOI testing are incubation conditions and humidity. The amount of humidity must be kept constant to an appropriate level as the agar should not be allowed to dry out. Drying out might affect the growth of bacteria as well as the diffusion of the antimicrobials.
5.1 Humidity Control
The high level of humidity will lead to the accumulation of excessive moisture on the agar’s surface, resulting in possible dilution of the antimicrobial agent and the development of larger zones of inhibition. Low humidity will dry the agar and may influence the viability and growth patterns of the bacteria. Humidity in the incubators should be under control for assured conditions during ZOI testing.
5.2 Atmospheric Composition
This is because some bacteria are very specific to the atmospheric conditions under which they can grow to their best. For instance, anaerobic bacteria require an environment that is free of oxygen. Capnophilic bacteria require higher concentrations of carbon dioxide. Applying the proper incubation conditions for specific bacterial species permits an appropriate assessment of the activity of antimicrobials.
6. Duration of Incubation
Another factor to be considered in terms of the influencing factors to the outcome of ZOI testing is the incubation time. The standard time used while the ZOI tests are in their incubation stage is 16-18 hours. Any variations from this standard come with fluctuations of the results.
6.1 Over- and Under-Incubation
Over-incubation may allow for bacterial regrowth in inhibition zones, resulting in a low measurement that underestimates the efficacy of an antimicrobial while the under-incubation may lead to large inhibition zone irrespective of the activity of antimicrobials that overestimates the efficacy of antimicrobial It is therefore important that adhering to the recommended incubation times is kept to in order to get accurate and dependable results.
7. Antimicrobial Disk Quality
Correct results in ZOI testing can be obtained only if quality and storage conditions of antimicrobial disks are strictly adhered to. Disks should be stored according to the manufacturer’s guidelines so that their potencies can be maintained.
7.1 Disk Potency and Storage
The activity of antimicrobial disks may get depleted with time, particularly if the storage conditions are poor. Degradation of active compounds may result from high moisture, light, or temperature. This compromises the reliability of ZOI measurements. Fresh disks that are well stored should be used in testing to give valid test results.
7.2 Consistency of Disk Application
The disks containing antimicrobials should also be applied consistently across the agar surface. Apply the disks uniformly and press them with a soft pressure to achieve good contact with agar. Variations in disk application may lead to an uneven pattern of diffusion and varied inhibition zones.
Conclusion
Major determinants of the precision and reliability of results of Zone of Inhibition (ZOI) tests are environmental factors. Temperature, pH, composition, and depth of agar, inoculum density, humidity, incubation conditions, and the quality of antimicrobial disks significantly impact the outcomes of such tests. The understanding and control of these factors are very basic in obtaining the data consistently and meaningfully based on which the assessment of the efficacy of the tested antimicrobials is done. Standardization of these environmental conditions can thus ensure validity to the results of ZOI testing in laboratories and ultimately aid in devising and optimizing effective antimicrobial treatments.
Ensure your antimicrobial agents are tested under precise and controlled environmental conditions with Microbial Investigations Switzerland (MIS). Our expert team and state-of-the-art laboratories are dedicated to delivering accurate and dependable Zone of Inhibition (ZOI) testing results. Trust MIS to validate the efficacy of your products and help you achieve continuous improvement and innovation.
Contact us today to learn more about our comprehensive testing and validation services. Together, let’s advance the science of antimicrobial protection.