- Swiss testing laboratory
BS EN 17915 : 2022
Test method for the evaluation of virucidal activity of chemical disinfectants on hard non-porous surfaces in food, industrial, domestic, and institutional areas (phase 2 step 2)

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Related tests for you
Evaluation of virucidal activity for instruments used in the medical area
Evaluates the virucidal activity of disinfectants used in medical areas on non-porous surfaces without mechanical action.
Quantitative non-porous surface test for evaluating the virucidal activity of disinfectants used in the veterinary area.
Quick understanding of the test
BS EN 17915 : 2022 - Test method for the evaluation of virucidal activity of chemical disinfectants on hard non-porous surfaces in food, industrial, domestic, and institutional areas (phase 2 step 2)
Application
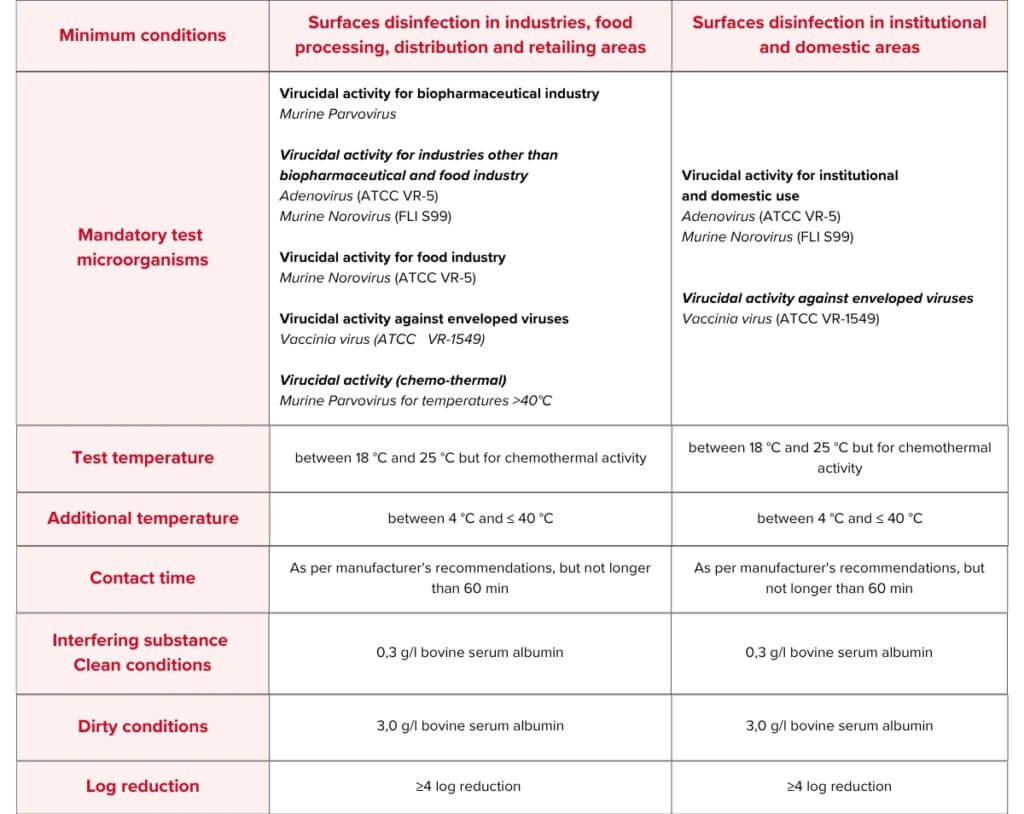
- Murine Norovirus
- Adenovirus
- Vaccinia Virus
- Murine Parvovirus
- Test virus suspension with interfering substance is inoculated onto a test surface and dried.
- Dried inoculated surface is exposed to disinfectant for specific contact times.
- At the end of contact time, the disinfectant activity is neutralized.
- Infectious titre of virus recovered is determined and compared with infectious titre from control test.
- Ensures the development of innovative disinfectant formulations tailored to meet high standards of virucidal activity.
- EN 17915 validates disinfectants for diverse areas such as food preparation, industrial facilities, domestic spaces, and institutional environments.
Turnaround Time
Passing criteria
Do you have a product that needs testing?
Abstract
EN 17915 standard specifies the test method to determine virucidal activity of disinfectants on non – porous surfaces without mechanical action. EN 17915 test is applicable to disinfectant products used in food, industrial, domestic, and institutional areas, except for the situations where disinfection is medically indicated and excluding products used on living tissues.
EN 17915 is a carrier test method (phase 2, step 2) that aims at determining the efficacy of disinfectants under more realistic conditions.
EN 17915 Test Conditions & Requirements
EN 17915 Test Method
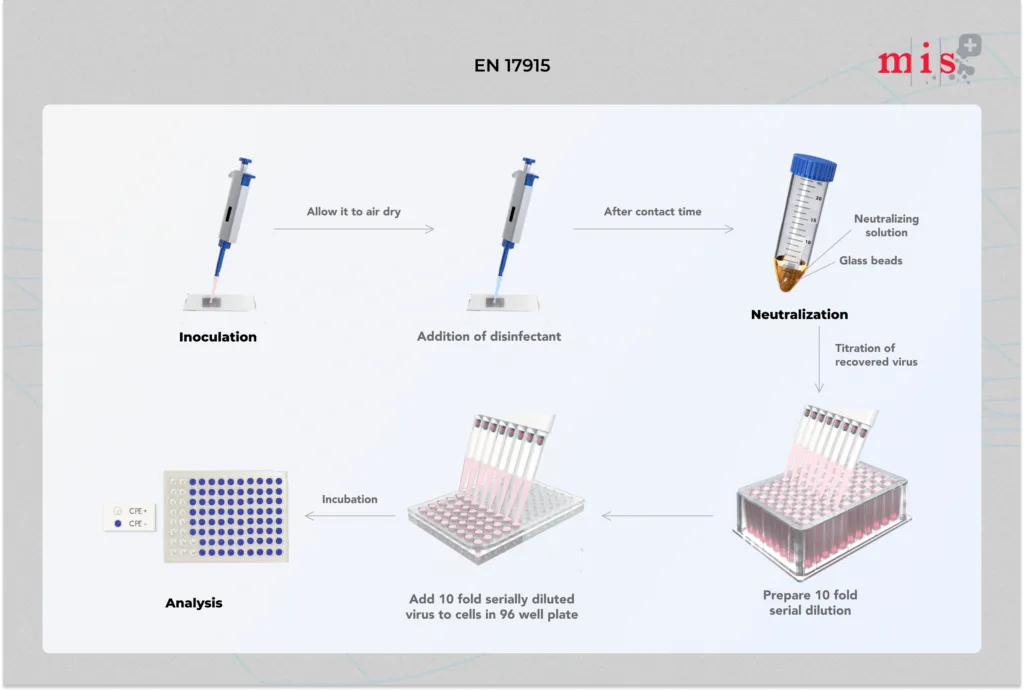
- Test suspension of virus with interfering substance is inoculated onto a test surface and dried out.
- Disinfectant sample is evenly spread on dried microbial film and allowed to interact for specific contact times. In parallel, a control test is run wherein the virus infected test surface is treated with hard water instead of disinfectant.
- At the end of contact time, the test surface is immediately transferred to the cell maintenance medium to neutralize further activity of the disinfectant sample.
- Infectious titre of virus recovered in cell maintenance medium is determined and compared with infectious titre from control test.
Passing criteria
The disinfectant product must demonstrate at least 4-log reduction to pass the standard.
Importance of EN 17915 Test
Many viruses exhibit the ability to survive on hard surfaces for an extended period and remain infectious. The process of disinfection plays a crucial role in elimination of harmful viruses and ensures a safe and hygienic environment. Not all available disinfectants are capable of effectively killing viruses. It is essential to use disinfectants formulated with virucidal agents for effective virus disinfection.
As per European norms, disinfectants claiming virucidal activity must be tested for their efficacy under standardized methods. The European Standard EN 17915 has been devised to check the virucidal efficacy of non-porous surface disinfectants before their intended applications.
Conclusion
MIS performs routine virucidal testing on disinfectants and antiseptics utilized for diverse application areas including food, industrial, domestic, institutional, medical, and veterinary settings.
Our cutting-edge facility offers antiviral efficacy research globally, catering to products with antiviral potential.
Explore, EN 16777 test for virucidal activity of chemical disinfectants at MIS lab.
To get a quote on EN 17915 test or to learn more about our testing capabilities, please contact our experts today.
Frequently Asked Questions

DR. Martinoz Scholtz
EN 17915 describes phase 2, step 2 test method to assess virucidal efficacy of non-porous surface disinfectants utilized in food, industrial, domestic, and institutional areas. The EN 17915 test method stimulates more real conditions by using a test surface (career) to determine the virucidal potential of disinfectant samples.
EN 17915 test is applicable to products used for disinfection of non-porous surfaces. These products are commonly used across various sectors such as food, industry, households, and institutions.
EN 17915 test takes 4-5 weeks to complete.
At Microbe Investigations Switzerland, we test for EN 17915 using the following microbial strains: Adenovirus (ATCC VR-5), Murine Norovirus (FLI S99), Vaccinia virus (ATCC VR-1549), and Murine Parvovirus. Additional virus strains can be tested on customer’s request.
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
Did you know there are
Antibacterial testing of disinfectants plays
Antimicrobial testing is important to
Antibacterial efficacy testing is an
Let’s face it, we are