- Swiss testing laboratory
BS EN 14562:2006
Quantitative carrier test for the evaluation of fungicidal or yeasticidal activity for instruments used in the medical area (phase 2, step 2)

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Related tests for you
Quick understanding of the test
BS EN 14562:2006 - Quantitative carrier test for the evaluation of fungicidal or yeasticidal activity for instruments used in the medical area (phase 2, step 2)
Application
- Candida albicans (ATCC 10231)
- Aspergillus niger (ATCC 16404)
- Test microbial suspension with an interfering substance is inoculated onto a glass surface (carrier) and dried.
- Inoculated carrier is immersed in the disinfectant solution and incubated.
- At the end of contact times, the carrier is transferred into a neutralizing solution followed by plating and incubation to determine the number of viable cells present in the sample.
- Helps manufacturers in the improvement of fungicidal and yeasticidal products.
- Verifies efficacy of disinfectants under practical application conditions.
Turnaround Time
Passing criteria
Do you have a product that needs testing?
Abstract
EN 14562 specifies test conditions and efficacy criteria for chemical disinfectants and antiseptics claiming fungicidal or yeasticidal properties. This standard is applicable to products intended to be used for instrument disinfection in medical areas.
EN 14562 is a (phase 2, step 2) carrier test. The aim of the test is to simulate real-world conditions to evaluate the effectiveness of disinfectants for their intended use. EN 14562 test is performed by selecting a suitable carrier material that closely resembles the type of surfaces used in practical settings.
EN 14562 Test Conditions & Requirement
- Mandatory test microorganisms
- Fungicidal activity – Candida albicans (ATCC 10231), Aspergillus niger (ATCC 16404) (formerly Aspergillus Brasiliensis)
- Yeasticidal activity – Candida albicans (ATCC 10231)
- Test temperature – 20 °C
- Contact time – 60 mins
- Interfering substance
- Clean conditions – 0.3 g/l bovine albumin solution
- Dirty conditions- 3.0 g/l bovine albumin solution + 3.0 ml/l washed sheep erythrocytes
- Log reduction – Test product must demonstrate at least 4 log reduction values against mandatory test microorganisms
EN 14562 Test Method
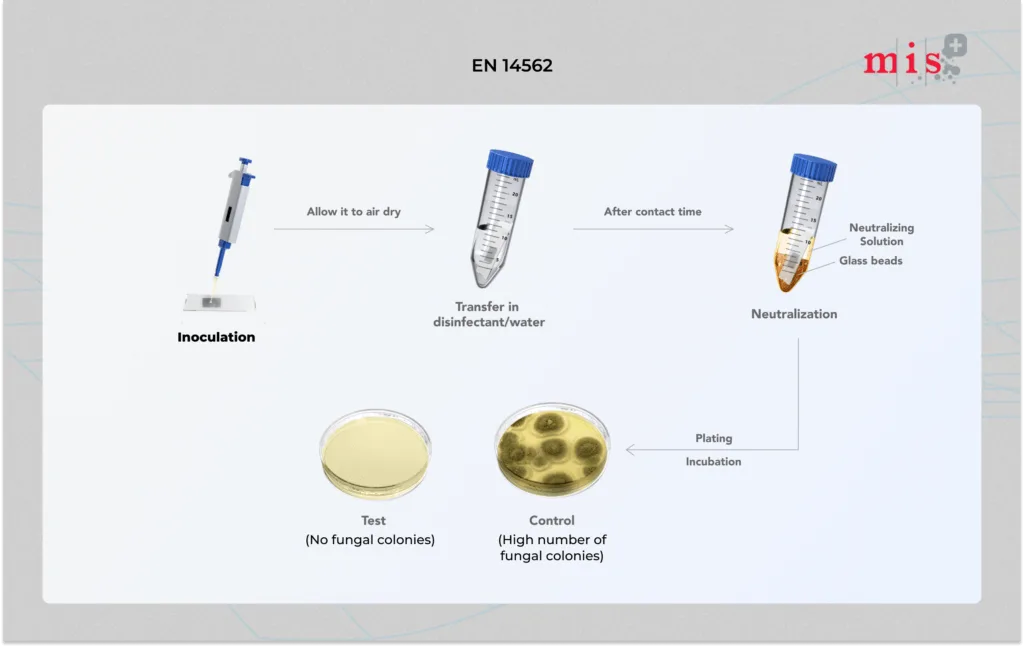
- A test suspension of microorganisms (yeast or fungus) with an interfering substance is inoculated onto a glass surface (carrier). This is left to dry naturally.
- Inoculated carrier is immersed into disinfectant solution and maintained at 20 °C for 60 mins. In parallel, a control test is run where the glass carrier is immersed in hard water (control sample) instead of disinfectant.
- At the end of contact times, the carrier is transferred into a neutralizing solution containing glass beads. The solution is shaken to separate yeast/ fungal spores from the glass surface.
- Neutralizing solution is then plated and incubated to determine the number of viable cells present in the sample.
- Microorganisms from both treated carrier and control test (carrier treated with hard water instead of disinfectant) are enumerated and reduction in microbial count is calculated.
Importance of EN 14562 Test
In hospital environments, Candida and Aspergillus are found responsible for causing opportunistic infections, especially in immunocompromised patients. One of the major contributing factors to these infections is improper sterilization and disinfection of medical equipment, instruments, and devices.
Therefore, disinfection of instruments is regarded as a crucial hygiene practice in healthcare settings to prevent fungal infections.
In Europe, regulatory approval is mandatory for manufacturers/companies of medical instrument disinfectant products before market release of their products. EN 14562 standard outlines conditions and test parameters to assess efficacy of disinfectant products against Candida albicans and Aspergillus niger.
Conclusion
Looking for EN 14562 testing services, MIS experts would be happy to help you.
At MIS, we specialize in providing tailored testing solutions for disinfectant/antiseptic products that are utilized in various sectors including medical, veterinary, domestic, and other applications.
With our state-of-the-art laboratory infrastructure and a team of industry experts, we have developed a comprehensive test analysis program to ensure accurate and reliable results.
Additionally, we perform EN 14563 test to evaluate mycobactericidal or tuberculocidal activity of medical instrument disinfectants
To get a quote on the EN 14562 test or to seek expert consultation from our microbiology experts, contact us today.
Frequently Asked Questions

DR. Martinoz Scholtz
EN 14562 is a (phase 2, step 2) carrier test to assess fungicidal or yeasticidal activity of medical instrument disinfectants. In order to comply with EN 14562 standard, test product must demonstrate at least a 4 log reduction in the number of test microorganisms.
EN 14562 test is applicable to disinfectants and antiseptics used for the instruments disinfection in medical areas.
EN 14562 test takes 3 – 4 weeks to complete.
At Microbe Investigations, we test for EN 14562 using the following microbial strains – Candida albicans (ATCC 10231), and Aspergillus niger (ATCC 16404).
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
The selection of a suitable
Fungicidal disinfectant testing plays an
Fungi are found in diverse
Fungal contamination is a major
As viral outbreaks have become







