Home / Disinfectant Testing / antibacterial / EN 1500
- Swiss testing laboratory
BS EN 1500: 2013

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Related tests for you
Quick understanding of the test
BS EN 1500: 2013 - Chemical disinfectants and antiseptics — Hygienic hand rub — Test method and requirements (phase 2/step 2)
Application
- Escherichia coli K12
- Volunteers’ hands are pre-washed to remove impurities and then immersed in a microbial suspension until dry.
- Fingertips are dipped into tryptic soy broth (TSB) to establish pre-contamination levels of viable bacteria.
- Hands are then rubbed with a sample of the handrub product for a predetermined contact time.
- After the contact time, fingers are immersed in a neutralizing solution to recover any remaining viable bacteria.
- A small sample of the neutralizing extract is plated and the bacteria are enumerated.
- Provides a standardized test to evaluate the efficacy of hygienic hand rubs in reducing transient microorganisms.
- The test simulates real-world usage conditions, making the results highly relevant and practical.
Turnaround Time
Passing criteria
Do you have a product that needs testing?
Abstract
The EN 1500 is a crucial standard applied to test the efficacy of products intended for use as hygienic hand rubs. These products are tested to ensure effective personal sanitization in high-risk areas such as hospitals, clinics, and other medical institutions. This standard aims to ensure that hand rub products significantly reduce transient microorganisms on users’ hands, thereby reducing the risk of infection spread.
EN 1500 Test Method Conditions & Requirements
The test method evaluates the efficacy of hand rub products under simulated practical conditions. The standard specifies the following conditions and requirements for testing:
- Mandatory test organisms
The primary organism used in testing is Escherichia coli K12.
- Subjects
The test requires 18-22 volunteers whose hands must be free of abrasions and cuts.
- Contact times
The contact time for the hand rub product ranges between 30-60 seconds or as recommended by the manufacturer.
- Reference product
Propan-2-ol (Isopropyl alcohol, IPA) is used as the reference product at 60% concentration by volume.
EN 1500 Test Procedure
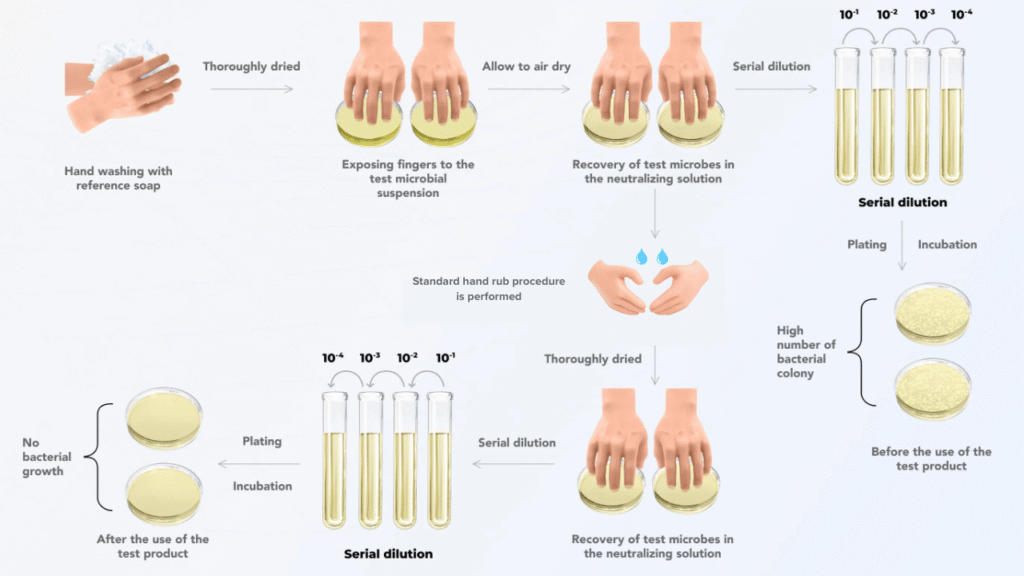
Pre-wash
- Volunteers wash their hands with non-antimicrobial soap and water, then dry them with sterile paper towels.
Contamination
- Hands are immersed in an E. coli suspension for 5 seconds and then air-dried for 3 minutes.
Baseline sampling (initial value)
- Fingertips are rubbed over the base of the thumb of the other hand in a standard manner for 30 seconds using sample liquid (neutralizer + TSB). The sampling liquid is then pipetted into a sterile container.
Application of test product
- The test product is applied according to the manufacturer’s instructions, ensuring thorough coverage and rubbing for a specified time, usually 30 seconds.
Post-treatment sampling (immediate value)
- Immediately after rubbing, the baseline sampling process is repeated.
Incubation and enumeration
- An aliquot of the sample is taken, neutralized in a specified volume of neutralizing solution, plated onto TSA plates, and incubated at 36±1°C for 24±2 hours, followed by the counting of microbial colonies.
Reference procedure
- This procedure is repeated using the reference product (60% v/v propan-2-ol) with the same volunteers.
Neutralization and control
- Proper neutralization of the test product must be ensured by including neutralizer effectiveness controls.
Acceptance criteria
- The test product should demonstrate at least an equivalent or superior reduction in bacterial count compared to the reference product.
Benefits of EN 1500 certification for manufacturers
Enhanced Credibility:
- Certification improves brand reputation and credibility by validating the efficacy of products, providing assurance from an accredited authority that European standards are met. This instills confidence in users, healthcare professionals, and authorities that the product is effective in eliminating harmful pathogens.
Regulatory Compliance:
- Compliance with European norms ensures increased access to and acceptance by the EU market. Certification helps meet legal requirements and proves the manufacturer’s dedication to safety and quality.
Improved Safety:
- Certified products help minimize the risk of contamination spread between medical personnel and patients. Certification ensures that the hand rub effectively eliminates microbial flora, significantly reducing HAIs and safeguarding health in facilities where infection control is paramount.
Competitive Advantage:
- EN 1500 certification serves as a recognizable mark indicating that the product has undergone rigorous testing to prove its efficacy and safety. This differentiation is crucial for healthcare institutions, businesses, and consumers when selecting hand hygiene products.
Customer Satisfaction and Loyalty:
- Achieving high standards of safety and efficacy raises customer satisfaction and loyalty, especially in the health sector, translating to long-term relationships with hospitals, clinics, and healthcare providers who prioritize patient safety and infection control.
How can MIS help?
At Microbe Investigations Switzerland (MIS), the EN 1500 hand rub testing is performed by experienced professionals using advanced analytical techniques. Compliance ensures that hand rub products are effective in practical applications, providing a high level of protection against the transmission of infections.
For more information about our EN 1500 testing services or to request a quote, connect with our experts at MIS today. Ensure your hand rub products meet the highest standards of efficacy and safety with our comprehensive testing solutions.
Frequently Asked Questions

DR. Martinoz Scholtz
The EN 1500 test simulates practical conditions to evaluate if hygienic hand rubs effectively eliminate transient microflora when used on artificially contaminated hands of volunteers.
The test applies to hand rub products used in hospitals, community medical facilities, dental settings, clinics of schools, kindergartens, and nursing homes.
The test takes 2-3 weeks to complete.
At Microbe Investigations, we perform the EN 1500 test using Escherichia coli (ATCC 8739). Additional strains can be added upon customer’s request.
This test is a European standard specifically developed for hygienic hand rubs. Unlike other tests focusing on different pathogens or conditions, this test quantifies the log reduction of transient microbial flora on hands under simulated real-life conditions. EN 1500 is unique as it involves human volunteers, ensuring effective performance in actual usage scenarios.
To pass, a hand sanitizer must demonstrate a statistically significant reduction in microbial flora comparable to a reference solution of 60% propan-2-ol, achieving effectiveness under the test conditions.
Prioritizing EN 1500 compliance ensures the hand sanitizer effectively reduces transient microbial flora, providing high standards of hygiene and safety. Compliance enhances product credibility and marketability, particularly in the European market where this standard is widely recognized and trusted.
The test is primarily designed for alcohol-based hand rubs, the most commonly used hand sanitizers. While other formulations can be tested, they need comparison against the standard reference solution of 60% propan-2-ol, which may not always be relevant for non-alcohol-based products.
Testing should be conducted at the initial development and formulation stage. Re-testing may be necessary in case of substantial formulation changes or processes. Periodic testing ensures continuous compliance and effectiveness, especially during regulatory reviews or market audits.
Common reasons for failure include insufficient alcohol content, poor formulation, weak application method, or incorrect volume usage during testing. Production fluctuations or ineffective dispersion of active ingredients can also lead to failure.
The test is crucial in maintaining public health and hygiene, ensuring hand sanitizers effectively reduce microbial load on hands, consequently preventing infection spread. Products following this standard contribute to infection prevention in healthcare facilities, food processing industries, and public environments.
To prepare, ensure the product has the appropriate alcohol concentration (typically 60-95% ethanol or isopropanol). Conduct thorough pre-testing to verify efficacy, adhere to precise formulation and production standards, and consult experts in microbiological testing. Conducting pilot studies can help identify and rectify potential issues before formal testing.
Benefits include increased customer confidence, compliance with regulatory bodies, and competitive advantage. Certification assures regulatory agencies and customers of product effectiveness, leading to increased sales and market acceptance globally.
Sanitizer formulations with high alcohol content like 60%-95% ethanol or isopropanol are more likely to pass this test. Formulations which include skin conditioners and emollients can improve user compliance and effectiveness by ensuring the products are safe to use and do not cause any skin irritation on frequent usage.
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
Did you know there are
Antibacterial testing of disinfectants plays
Antimicrobial testing is important to
Antibacterial efficacy testing is an
Let’s face it, we are