- Swiss testing laboratory
ISO 21702 Test

Hassle-free testing experience
Need to get a product tested? No worries! To and fro logistics are on us; we collect your products, test them and, deliver them back to you.
Related tests for you
Quick understanding about test
ISO 21702 Test - Measurement of Antiviral Activity on Plastics and other Non-Porous Surfaces
- Influenza A virus ( H3N2)
- Feline calicivirus
- Control and uncontrolled samples are prepared according to guidelines and separated to avoid cross-contamination.
- Viral inoculum (400 µL) is applied, covered with a coverslip, and evenly spread.
- The neutralizing agent is immediately added to control samples after inoculum application.
- Samples are incubated at 25°C and 90% humidity for 24 hours.
- The remaining virus is recovered using neutralizing broth, followed by serial dilution.
- Viral titer is quantified using Plaque Assay or TCID50 to assess infectivity.
- Confirms the antiviral efficacy of materials for improved product reliability.
- Reduces viral transmission risks and enhances user safety.
Turnaround Time
It typically takes four weeks.
To pass the test, the log value of the number of plaques recovered immediately after inoculation from the treated test specimens must be lower than the untreated specimen.
Do you have a product that needs testing?
Abstract
ISO 21702:2019 test method is an important standard for assessing the effectiveness of non-porous products treated with antiviral agents, particularly those used in environments where viral contamination poses a significant risk to public health. As the demand for antiviral-treated products continues to rise, this standard provides manufacturers and product developers with a standardized means of confirming the validity of their materials.
The standard gives a robust framework by which the antiviral properties of plastic and other non-porous surfaces can be assessed. This test method is applicable to a wide range of antiviral-treated products, particularly used in healthcare and public spaces where the spread of viral infections must be minimized.
Scope of products for testing
The ISO 21702 test can be used to evaluate antiviral activity on plastics and other non-porous surfaces such as:
- Coating materials
- Metal substrates
- Ceramics
- Natural and artificial leather, rubber, etc.
ISO 21702 Test Conditions and Requirements
Test organism: Influenza A virus (H3N2), Feline calicivirus
Temperature: 25 ± 1℃
Relative humidity: ≥ 90%
Contact time: Typically 24 hrs or as per manufacturer’s instructions.
Sample preparation
Treated samples
Nine sterilized treated specimens are needed for the evaluation of antiviral activity.
- Three treated samples are used to estimate the infectious titer of the virus after a 24-hour contact period. This measures how effective the surface is in reducing the viral load.
- Six treated samples are maintained as controls to test the suppressive efficiency of the test material toward the host cells. The control samples ensure that there is no cytotoxic effect on the host cells, no reduction in the cell’s sensitivity to the virus, and any antiviral activity present in the neutralizing broth should be effectively inactivated.
Control samples
Twelve sterilized untreated control samples will be required to provide a baseline for comparison.
- Three untreated control samples are used to measure the infectious titer of the virus immediately post-inoculation. This provides a reference of the viral activity before contact with the treated surface.
- After having a 24-hour contact period, three untreated control samples are tested to assess the amount of virus that remains viable on an untreated surface.
- In addition, six untreated control samples are used to verify the suppressive efficiency of the antiviral-treated samples. Like treated samples, these controls test for the absence of cytotoxic effect, maintenance of cell sensitivity, and neutralization of antiviral activity.
ISO 21702 Test Methodology
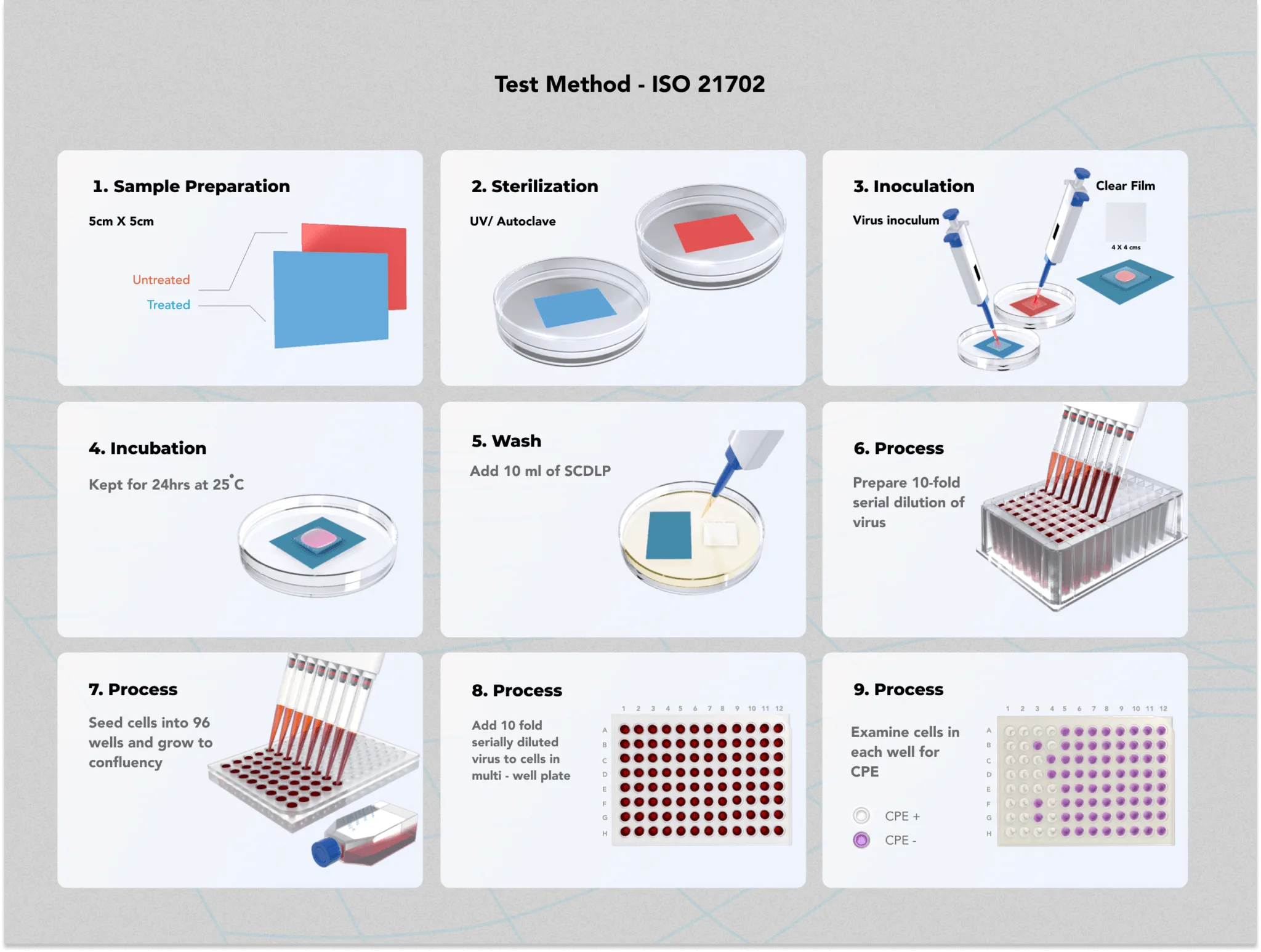
- Preparation of samples
Specimens are prepared as per the standard’s guidelines. Treated and untreated samples are kept separately in a sterile plate to avoid cross-contamination. - Application of test inoculum
A known volume of 400 µL of viral inoculum is spread over the surface of each sample. A coverslip is gently placed on the inoculum and pressed lightly to ensure that it spreads to the edges without leaking beyond the specimen. - Immediate neutralization for control samples
For the control (untreated) samples, 10 mL of SCDLP broth or another appropriate neutralizing agent is added immediately after the application of the inoculum. - Incubation
Treated and control samples are placed in the incubator at 25°C with a relative humidity of 90% for a contact period of 24 hours. The contact time may vary depending on the manufacturer’s instructions, but typically 24 hours serves as the common standard. - Recovery of virus after incubation
After incubation, 10 mL of neutralizing broth is added to both treated and control samples to recover any remaining active virus from the surface. The neutralizing solution is then serially diluted up to 10 dilutions. - Virus quantification
The infectious titre of the isolated virus is determined by using assays such as the Plaque Assay or TCID50 assay. These methods allow quantification of the virus and evaluate its ability to infect host cells after the 24-hour contact period. - Calculation of antiviral activity: The antiviral activity of the treated surface is determined based on the equation,
R = Log10(Ut) – Log10(At)
Where:
- R is the antiviral activity value.
- Log10(Ut) is the logarithmic average of the infectivity titer of the virus after the specific contact time with the untreated control specimen.
- Log10(At) is the logarithmic average of the infectivity titer of the virus after the specific contact time with the treated test specimen.
Passing criteria
To pass the test, the log value of the number of plaques recovered immediately after inoculation from the treated test specimens must be lower than the untreated specimen.
Importance of ISO 21702 test
ISO 21702 is a critical standard for manufacturers developing antiviral surface technologies, as it provides scientific validation of antiviral effectiveness. The test also supports regulatory compliance by helping manufacturers meet international requirements for antiviral-treated products, while offering robust data to substantiate antiviral efficacy claims in both marketing and technical documentation.
Conclusion
At Microbe Investigations, we conduct ISO 21702 testing on a wide range of plastic and non-porous surfaces, including coatings, metal substrates, ceramics, natural and synthetic leather, rubber, and more. Our team of skilled microbiologists and technical experts is dedicated to delivering high-quality assurance testing.
In addition, our state-of-the-art testing facilities offer comprehensive antimicrobial testing for textiles, coatings, and surface disinfectants.
For more information about our antimicrobial testing services, please contact our experts today.
Frequently Asked Questions

DR. Martinoz Scholtz
ISO 21702 test describes a test method to determine the antiviral activity on treated plastics and other non-porous surfaces against specified viruses.
The standard applies to plastics and other non-porous surfaces, such as coating materials, metal substrates, ceramics, natural and artificial leather, rubber, etc.
The turnaround time for this test is four weeks. We also offer a “Fast Track” program, where the turnaround time is 2-3 weeks depending on the virus strain.
At Microbe Investigations Switzerland, we test the antiviral efficacy using SARS CoV-2 (Omicron, Delta, Wuhan variant), Betacoronavirus 1 (OC43) (ATCC VR-1558), Human coronavirus 229E (ATCC VR-740), Influenza A (H1N1) (ATCC VR-1469), Influenza A (H3N2) (ATCC VR-1679), Human Poliovirus type 1 (LSc-2ab), Human Adenovirus type 5 (ATCC VR-5), Murine Norovirus (S99), Vaccinia virus (ATCC VR-1549), and Feline calicivirus (strain F-9).
The test involves applying a virus to a treated surface, incubating it, and measuring the reduction in viral activity compared to a control surface.
Key parameters of this test include the type of virus, concentration of the virus ( virus titer), incubation period, and the recovery of the virus after incubation.
While ISO 21702 is used to measure the antiviral activity, ISO 22196 tests for antibacterial activity on plastics and other non-porous surfaces. Both of the above standards offer a valid method for determining antimicrobial activity, but they differ based on the type of pathogen tested.
Meet the best of the blend of
R&D, Efficacy Testing,
Innovation and Passionate
Experts at MIS.





Explore More
Microscopic spores of fungi and
Face masks coated with antibacterial
Introduction The Japanese Industrial Standards
What is ISO 18184 Testing?